OVERVIEW
WHAT IS VERACITYDuring pregnancy, fetal DNA travels from the placenta to the mother’s blood stream and circulates along with her own DNA. VERACITY is a new generation non-invasive prenatal test that accurately measures the fetal cell-free DNA in the maternal blood to detect the presence of fetal aneuploidies and microdeletions. VERACITY has been validated for both single and twin pregnancies as well as pregnancies conceived through in-vitro fertilization (IVF).
The doctor or lab collects a blood sample from the mother’s arm and sends it to our state-of-the-art laboratories for analysis. At the laboratory, cell-free DNA is isolated from the mother’s blood and analyzed using our proprietary, new generation analytic and bioinformatic technology. Results are provided to your doctor in a few working days.

UNIQUE FEATURES OF VERACITY
VERACITY technology enables chromosomal aneuploidy detection as well as fetal fraction measurement with unparalleled accuracy
Targeted genomic analysis
VERACITY is designed to avoid genomic regions with complex architecture that affect test performance. This increases precision and accuracy.
High-read depth
VERACITY captures DNA fragments from targeted regions on chromosomes of interest and reads these at an extremely high read-depth, improving the statistical accuracy of the analysis and increases the sensitivity and specificity.
Read-depth is the number of times a nucleotide in the genome is read during analysis.
Fetal fraction measurement
A proprietary bioinformatics software accurately calculates fetal fraction which increases the robustness and reliability.
Multi-engine analysis pipelines
Proprietary bioinformatics pipelines analyze the sequencing data produced from each test. This multi-engine analysis increases the sensitivity and specificity of aneuploidy, microdeletion and fetal gender detection.
AUTOSOMAL ANEUPLOIDIES
● Down syndrome (Trisomy 21)
● Edwards syndrome (Trisomy 18)
● Patau syndrome (Trisomy 13)

TYPES OF CONDITIONS DETECTED BY VERACITY
Genetic conditions are caused by unwanted changes in the genome that happen during conception. Three types of genetic conditions are detected in Veracity:
- Autosomal aneuploidies: Genetic conditions that occur in chromosome pairs 1-22. Whole chromosome aneuploidies may refer to cases where chromosomes have an extra copy (trisomy) or a missing copy (monosomy).
- Sex chromosome aneuploidies: Genetic conditions that occur in chromosome pair 23, which defines gender. Women have two X chromosomes while men have one X and one Y chromosome. Sex chromosome aneuploidies occur when there are extra copies of the X or Y chromosome, or a missing copy of the X chromosome.
- Microdeletions: Genetic conditions caused by the loss of a part of a chromosome. Microdeletions are characterized by congenital abnormalities and intellectual impairment. The severity of the symptoms varies depending on the size and location of the microdeletion.
| ANEUPLOIDIES | |
| CONDITION | CAUSE |
| Down syndrome (Trisomy 21) | Three copies of chromosome 21 |
| Edwards syndrome (Trisomy 18) | Three copies of chromosome 18 |
| Patau syndrome (Trisomy 13) | Three copies of chromosome 13 |
| Turner syndrome (Monosomy X) | One chromosome X |
| Triple X syndrome (Trisomy X) | Three copies of chromosome X |
| Klinefelter syndrome (XXY) | Extra copy of chromosome X |
| Jacobs syndrome (XYY) | Extra copy of chromosome Y |
| XXYY syndrome | Extra copies of chromosomes X and Y |
| MICRODELETIONS | |
| CONDITION | CAUSE |
| DiGeorge syndrome (22q11.2) | Deletion of part of chromosome 22 |
| 1p36 deletion syndrome | Deletion of part of chromosome 1 |
| Smith-Magenis syndrome (17p11.2) | Deletion of part of chromosome 17 |
| Wolf-Hirschhorn syndrome (4p16.3) | Deletion of part of chromosome 4 |
CLINICAL PERFORMANCE
VERACITY is a new generation non-invasive prenatal test (NIPT) for the detection of fetal chromosomal aneuploidies and microdeletions. It uses proprietary technology based on cutting-edge research and development in molecular genetics and bioinformatics. It was specifically designed to avoid technical limitations and shortcomings of other NIPT.
VERACITY uses novel Targeted Enrichment Technology that enables with unparalleled accuracy chromosomal aneuploidy detection as well as fetal fraction measurement. Targeted regions on selected chromosomes and chromosomal regions are captured, enriched and analysed for the detection of aneuploidies and microdeletions using our proprietary genomic and bioinformatic technologies.
Validation studies have been conducted and are available (see table on the left).
| AUTOSOMAL TRISOMIES | |||||
| KARYOTYPE | NUMBER | FOLLOW-UP | CORRECT | SPECIFICITY | NPV/PPV |
| Normal | 10280 | 10280 | 10280 | 99.98% (99.93-99.998%) | 100% (99.96 – 100%) (NPV) |
| Trisomy 21 | 126 | 44 | 44 | 100% (92 – 100%) | 100% (92 – 100%) (PPV) |
| Trisomy 18 | 24 | 10 | 10 | 100% (69 – 100%) | 100% (69 – 100%) (PPV) |
| Trisomy 13 | 16 | 7 | 5 | 100% (48 – 100%) | 71% (29 – 96%) (PPV) |
| SEX CHROMOSOME ANEUPLOIDIES | |||||
| KARYOTYPE | NUMBER | FOLLOW-UP | CORRECT | SPECIFICITY | NPV/PPV |
| Normal | 6200 | 6200 | 6200 | 99.95% (99.86 – 99.99%) | 100% (99.94 – 100%) (NPV) |
| 45, X | 16 | 7 | 4 | 100% (40-100%) | 57% (18-90%) (PPV) |
| 47, XXX | 6 | 2 | 2 | – | – |
| 47, XXY | 10 | 4 | 4 | – | – |
| 47, XYY | 3 | 0 | – | – | – |
| 48, XXYY | 1 | 1 | 1 | – | – |
Kypri et al. Molecular Cytogenetics (2019) 12:34
MORE ABOUT PRENATAL TESTS
WHAT IS A PRENATAL TEST?It is a test that a pregnant woman can take to check for genetic conditions of the fetus before birth.
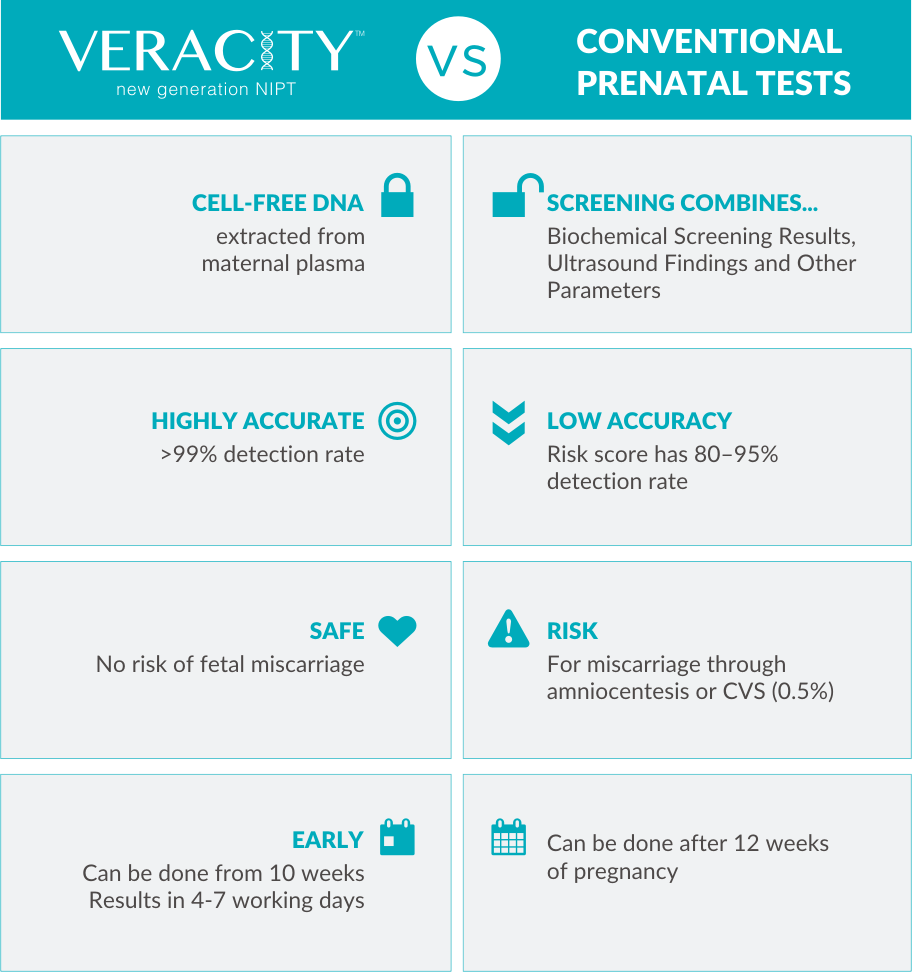
During the first trimester, a screening test will be offered which includes an ultrasound and a biochemical blood test. In combination with other parameters such as the mother’s age, the risk of specific genetic conditions occurring in the fetus is estimated without actually testing fetal DNA. The accuracy of this screening test is low (80 – 95%).
If the prenatal screening indicates that the fetus has a high risk of having a genetic condition, an invasive test will be recommended such as amniocentesis or chorionic villus sampling. These invasive tests are highly accurate (>99%) but they have approximately 1 in 200 chance of causing a miscarriage.
Now, with VERACITY, there is a highly accurate (>99%) and safe non-invasive prenatal test.

IS THERE AN AGE LIMIT FOR VERACITY?
No. All pregnant women of any age or risk category can do the VERACITY test. Although the frequency for such genetic conditions rises with the mother’s age, they can happen in any pregnancy.
Your doctor will inform you about the level of your risk.

HOW EARLY IN THE PREGNANCY CAN VERACITY BE PERFORMED?
VERACITY can be performed as early as the 10th week of a pregnancy to quickly obtain accurate results!
Be at least 10 weeks pregnant
Ask your doctor about taking VERACITY
Visit your doctor to have a single blood draw
We will analyze the blood sample in our laboratories
We will send your results to your doctor in 4-7 working days

2x 10 ml blood in BCT cell free DNA tubes
4-7 working days from sample receipt to the laboratory
| ANEUPLOIDY | SENSITIVITY | SPECIFICITY | PPV | NPV |
| Trisomy 21 | 99.12% (95.44-99.98%) | 99.99% (99.97-99.99%) | 96.60% (91.50-99.06%) | 99.998% (99.99-100%) |
| Trisomy 18 | 97.44% (86.52-99.93%) | 99.99% (99.98-99.99%) | 92.68% (80.08-98.46%) | 99.998% (99.99-100%) |
| Trisomy 13 | 100% (71.51-100%) | 99.99% (99.99-100%) | 86.67% (59.54-98.34%) | 100% (99.99-100%) |
| Sex Chromosome | 91.30% (71.96-98.93%) | 99.98% (99.97-99.99%) | 84.00% (63.92-95.46%) | 99.997% (99.99-100%) |
| Microdeletions | 100% (69.15-100%) | 99.99% (99.98-100%) | 88.89% (51.75-99.72%) | 100% (99.99-100%) |
Internal data based on 60,000 samples.
| Type of Sample | Number of Samples | Correct Call | Confidence Interval |
| Normal | 538 | 538 (100%) | 99.9 — 100 |
| T21 | 52 | 52 (100%) | 93.2 — 100 |
| T18 | 16 | 16 (100%) | 79.4 — 100 |
| T13 | 5 | 5 (100%) | 47.8 — 100 |
| Male | 244 | 244 (100%) | 99.9 — 100 |
| Type of Sample | Number of Samples | Correct Call | Confidence Interval |
| Normal | 73 | 73 (100%) | 95.1 — 100 |
| T21 | 24 | 24 (100%) | 85.8 — 100 |
| Type of Sample | Number of Samples | Correct Call | Confidence Interval |
| Normal | 286 | 286 (100%) | 99.9 — 100 |
| Sex Chromosome Aneuploidies | 14 | 14 (100%) | 93.2 — 100 |
Next generation sequencing (Illumina)
Proprietary Target Capture Enrichment Technology (Click here to see our Publications)
VEGA (proprietary analysis software)
POLARIS (proprietary analysis software)
hg19, NCBI GRCh37
>30 (precision 99,9%)
*Our tests can be ordered through our local partners. Please choose one of the locations listed below