OVERVIEW
WHAT IS ADVENTIA?Adventia is a new genetic test for carrier screening to determine whether a phenotypically healthy person is a carrier of a genetic disease. The genetic insight provided by Adventia can inform, guide and empower people on their reproductive choices and minimize the risk of people who are carriers transmitting a genetic disease to their children. Adventia offers a choice of different panels and testing for up to 229 moderate to severe autosomal recessive and X-linked genetic diseases.
Anyone can carry certain mutations (genetic changes) in their body. Some mutations may have no effect on our health and development, while others can cause a genetic disease. When an individual has a mutation in one of their genes, but the mutation is not powerful enough to be expressed, that individual is a carrier of a recessive disease. Two carriers of the same recessive disease can have a child who is affected, if the child inherits the mutation from both of them.
As carriers are asymptomatic, they are unaware of their carrier status and the risk of passing a mutation to their children. In fact, many mutations for recessive diseases could be inherited via multiple generations without clinical manifestation. Unless you have been tested, it is impossible to know whether you are a carrier of a recessive disease.

WHO COULD BENEFIT FROM ADVENTIA
Adventia is a beneficial and comprehensive test for everyone based on a novel and powerful technology and can provide meaningful results in a short turn-around time to help you minimize your risk of transmitting a genetic disorder to your children.
Couples planning to start their
families and want to know
about their carrier status
Any individual or couple going through
assisted reproduction, including IVF
Sperm and oocyte donors,
and recipients of sperm or oocyte donation
Couples who are already pregnant and
want to know whether their child has
a risk of having a genetic disease
High-risk population groups
for specific diseases
People with a family history of a
genetic mutation

Any individual wishing to know more
about their genetic background
WHAT DOES ADVENTIA TEST FOR?
Adventia screens for autosomal recessive and X-linked diseases.
Carriers of recessive diseases have one healthy gene and one gene with the mutation.

Autosomal Recessive
Autosomal Recessive Diseases affect chromosome pairs 1 to 22.
If both parents are carriers, they have:
● 1 in 4 chance of having an unaffected child
● 1 in 2 chance of having a child who is also a carrier,
who has inherited the mutation from only one parent
● 1 in 4 chance of having an affected child, who has inherited mutations from both parents.
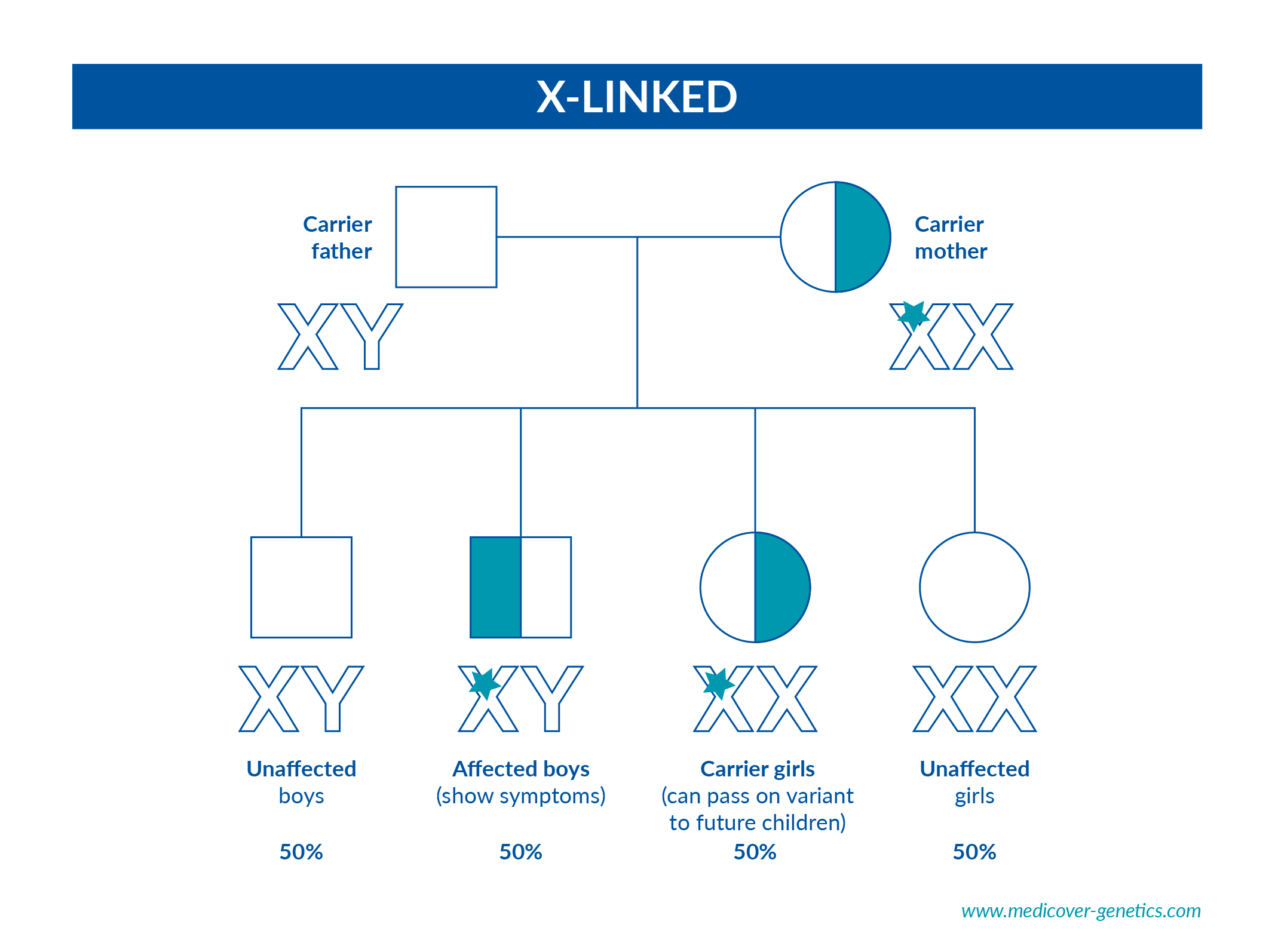
X-Linked
X-Linked Diseases affect the X chromosome, found on the 23rd chromosome pair
which determines gender.
● 1 in 2 chance of having a carrier daughter. Female carriers may or may not
exhibit disease characteristics due to X-inactivation*
● 1 in 2 chance of having an affected son. Males who have inherited the mutation are always affected, as they only have one X chromosome.
Adventia tests for autosomal recessive and X-linked diseases which have moderate to
severe phenotype, are high in carrier frequency, can compromise quality of life and
may be manageable through early interventions.








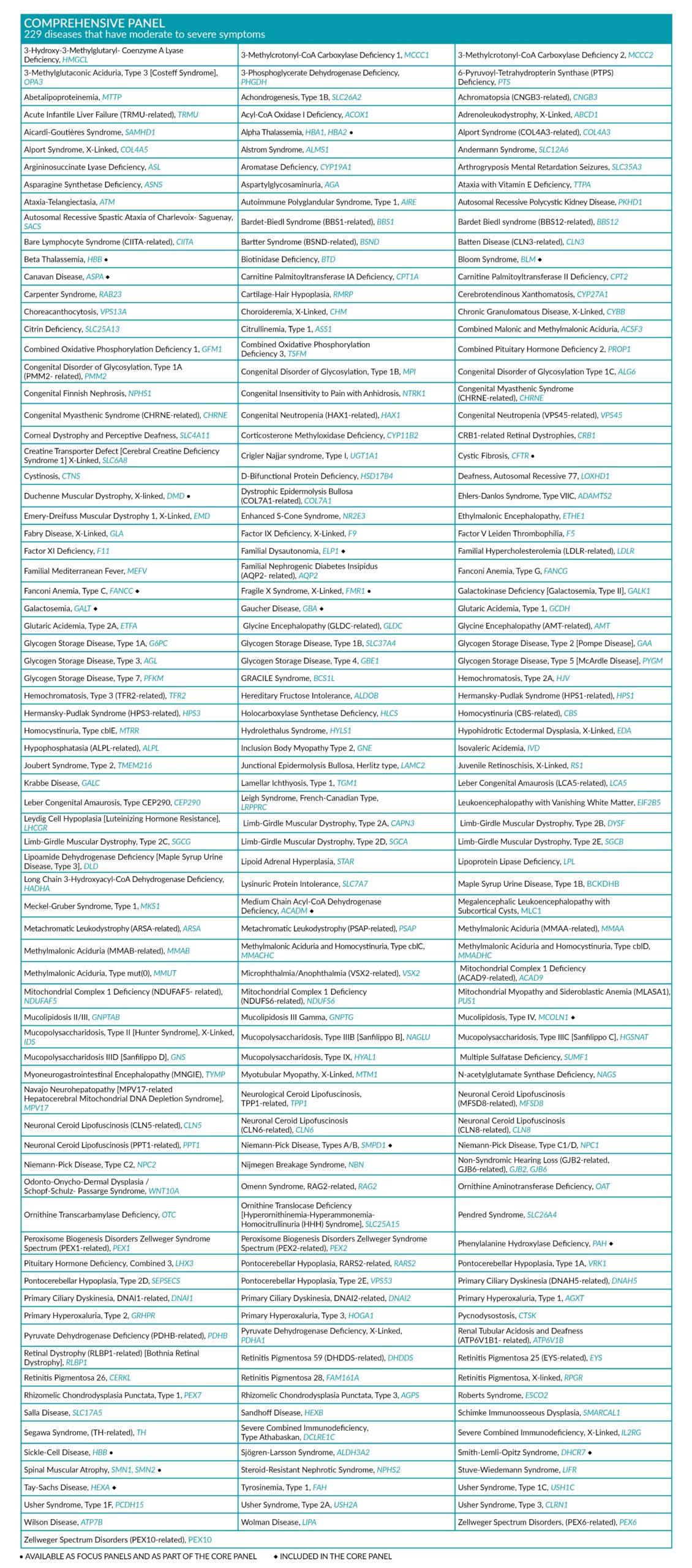
COMPREHENSIVE PANEL
229 diseases that have moderate to severe well-defined phenotype and high cumulative frequency. The Comprehensive panel includes all diseases of the Core panel, and covers a wide range of metabolic, cardiovascular and hematological diseases, amongst others.
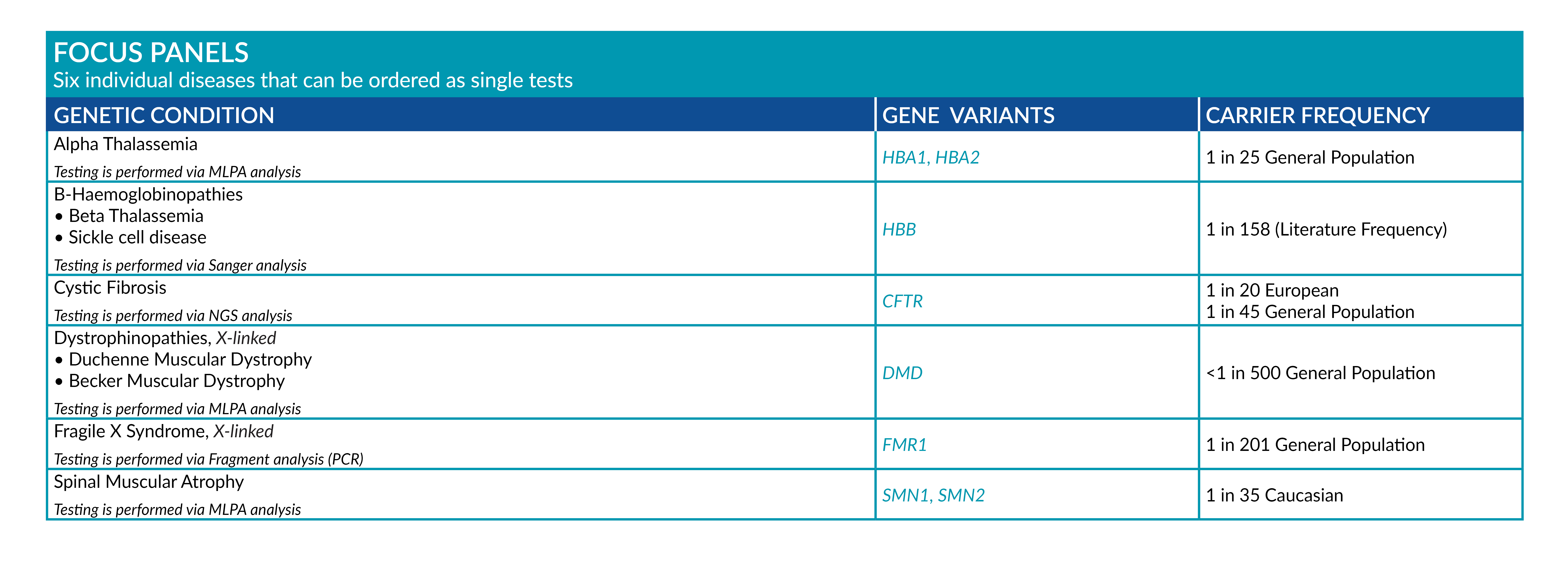
FOCUS PANELS
Six individual panels for highly frequent and severe genetic diseases:
● A-Thalassemia
● B-Haemoglobinopathies
● Cystic Fibrosis
● Duchenne Muscular Dystrophy
● Fragile X Syndrome
● Spinal Muscular Atrophy
CORE PANEL
20 genetic diseases of high incidence and clinical severity. The Core panel includes all diseases tested in the Focus panels, and other diseases including Phenylketonuria, Fanconi Anemia Group C and Tay-Sachs disease.