In June 2023, Medicover Genetics attended the 56th conference of the European Society of Human Genetics (ESHG) which took place in Glasgow, Scotland. The ESHG conference, which is organized annually, is one of the most prominent events in human genetics, with experts from around the world meeting to present and discuss advancements in the field.
Our team had the pleasure of attending and presenting our portfolio of genetic testing services and laboratory solutions, and scientists from across Medicover exhibited 8 posters presenting scientific and medical findings from our laboratories in the fields of reproductive health, oncology, and whole exome sequencing. These are summarized below.
Contents
- Molecular characterization of patients with gliomas using multi-gene next-generation sequencing panel
- Background/Objectives
- Methods
- Results
- Conclusion
- Whole exome sequencing in patients with developmental delay in routine diagnostics
- Background/Objectives
- Methods
- Results
- Conclusion
- Development of a liquid biopsy pan-cancer comprehensive genomic profiling assay with MSI and TMB immunotherapy biomarkers for therapy selection
- Background/Objectives
- Methods
- Results
- Conclusion
- A robust, accurate and cost-effective detection of alpha-thalassemia in an expanded multigene carrier testing
- Background/Objectives
- Methods
- Results
- Conclusion
- Novel variants in the PKD1 and PKD2 genes in German patients with autosomal dominant polycystic kidney disease
- Background/Objectives
- Methods
- Results
- Conclusion
- Development of a universal assay for multiple targeted NGS tests supported by a novel sample data management tool
- Background/Objectives
- Methods
- Results
- Conclusion
- Genetic diagnostic approach in nephrotic syndrome to decide on treatment strategy
- Background/Objectives
- Methods
- Results
- Conclusion
- Remarkably mild clinical presentation of classical Ehlers-Danlos syndrome (cEDS) in a three generation family due to a novel COL5A2 Glycine substitution
- Background/Objectives
- Methods
- Results
- Conclusion
Molecular characterization of patients with gliomas using multi-gene next-generation sequencing panel
Authors: A. Eliades, K. Tsangaras, A. Achilleos, C. Lemesios, O. Romanidou, P. Apostolou, C. Sotiriou, L. Constantinou, H. Kkoufou, M. Spyrou, S. Pissaridou, A. Matsentidou, M. Ioannides, G. Fountzilas, G. Koumbaris, P. C Patsalis
Background/Objectives
To study and determine the prevalence of somatic mutations in gliomas through a cost-effective multi-gene panel. Gliomas represent approximately one third of all central nervous system tumors. Although rare, gliomas have a high mortality rate, and a poor prognosis which depends on histology and grading.
Methods
48 patients with gliomas and available FFPE tissue samples were enrolled in the study, of which 32 proceeded with examination via a comprehensive 80-gene next generation sequencing (NGS)-based panel. 14 out of 32 tumor samples were further examined using a second panel of 55 genes to evaluate the accuracy and clinical utility of the 80-gene panel.
Results
- A total of 96 genetic alterations, including single nucleotide variants (SNVs), insertions and deletions (indels), and structural variants, were identified. 38 of those genetic alterations were scattered among 13 different genes.
- IHD1, PTEN, and TP53 pathogenic variants exhibited high incidence, with the latter having a 26.3% distribution of SNVs and indels.
- Among the structural variants detected, 15 patients had a CDKN2A deletion, followed by 10 patients with EGFR amplifications. These variants, whose detection was facilitated with the use of the 80‑gene panel, have potential for clinical utility.
- Our analysis also showed that patients with IDH1 mutant tumors had a statistically significant longer survival when compared to patients with IDH1 wild-type tumors.
- Regarding the evaluation of the 80- and 55-gene panels, high concordance (86.7%) was reached for the commonly targeted regions. A significant portion (91.6%) of additional variants that were identified by the comprehensive 80‑gene panel occurred in regions that were not targeted by the 55-gene panel, emphasizing the necessity of implementing a broader panel with additional targeted regions for glioma patients.
Conclusion
The application of comprehensive multigene testing enables the simultaneous identification of several genetic alterations in a wide range of genes across gliomas, improving molecular characterization and classification and providing further opportunities for tailored therapies.
Whole exome sequencing in patients with developmental delay in routine diagnostics
Authors: C. Albig, A. Munzig, C. Kurschat, J. Philippou-Massier, O. Wachter, S. Lott, K. Hörtnagel
Background/Objectives
To identify the genetic cause of neurodevelopmental disorders, such as autism spectrum disorders, language disorders, or intellectual disability, which affect 3% of children worldwide. Many neurodevelopmental disorders have a genetic cause, and several hundred genes have been reported to be associated with them.
Methods
Routine investigation procedure in our clinic involves initial genetic testing starting with chromosomal analysis, and if the patient’s results are negative, proceeding sequentially with array-CGH, fragile X analysis, and finally, whole exome sequencing (WES) preferably as a trio analysis. 488 patients with unresolved developmental delay were tested by WES in 2022.
Results
A likely pathogenic variant in genes associated with neurodevelopmental disorders was reported in 19.1% of cases, and a variant of uncertain significance (VUS) was identified in 14.3% of cases. In total, a variant was identified in 33.4% of cases.
- In 75.4% of cases, the variant was associated with an autosomal dominant disorder.
- In 51.2% of cases, the variant occurred de novo, whereas in 30.9% of cases the variant was inherited from a usually asymptomatic parent.
- Variants in the MECP2 gene, which is associated with Rett syndrome in females and X-linked intellectual disability syndrome in males, was reported in four cases.
- Variants in CHD2, CREBBP, MED13, and MED13L were repeatedly reported in three cases each.
Conclusion
WES is a useful diagnostic tool for patients with neurodevelopmental delay that can provide informative results in a timely manner. Trio analysis in particular can be helpful for evaluating the inheritance of the identified variant, thus avoiding the need for further family testing and providing useful information for future family planning.
Development of a liquid biopsy pan-cancer comprehensive genomic profiling assay with MSI and TMB immunotherapy biomarkers for therapy selection
Authors: K. Tsangaras, A. Eliades, C. Soteriou, S. Constantinou, A. Achilleos, C. Lemesios, C. Savva, C. Havadjia, C. Sotiriou, L. Constantinou, H. Kkoufou, M. Spyrou, S. Pissaridou, A. Matsentidou, C. Prokopi, M. Vaki, S. Georgiou, E. Kypri, M. Ioannides, G Koumbaris, P.C. Patsalis
Background/Objectives
To develop a highly accurate, non-invasive, NGS-based liquid biopsy assay which overcomes the limitations of established assays using FFPE samples, such as the limited availability of tissue biopsy material and the incomplete molecular profiling of the disease.
The assay, named ‘NeoThetis Pan Cancer Plus’ is a single assay that assesses genetic alterations (SNVs, indels, and rearrangements) in 221 genes that are clinically associated with approved targeted therapies and drugs under clinical and preclinical studies. The assay can also assess complex biomarkers such as microsatellite instability (MSI) and blood tumor mutational burden (bTMB) for therapy selection guidance in a wide spectrum of solid tumor malignancies.
Methods
The development and validation of the NeoThetis liquid biopsy assay is based on our proprietary technology:
- TACS preparation: synthetic DNA fragments, which are designed to capture DNA fragments from all coding regions of genes of interest
- Magnetic beads: streptavidin-coated micron-sized superparamagnetic beads provide the base for activating the TACS
- TACS activation: TACS are coupled to the magnetic beads to create the activated capture constructs
- DNA library preparation: DNA library is prepared by adding adapters to DNA fragments
- Target capture enrichment: Activated TACS and DNA library are mixed. DNA fragments from the regions of interest are captured
- Enriched library: The captured DNA fragments are eluted to create the enriched library
- Targeted sequencing and bioinformatics analysis: The enriched library is sequenced to very high read-depth and analyzed using bioinformatics.
Results
- The assay demonstrated 99% sensitivity of SNVs and indels with variant allele frequency (VAF) greater than 0.5%, and 100% sensitivity for CNAs and MSI.
- bTMB assessment showed high correlation when compared with reference material of known bTMB score.
Conclusion
Validation results demonstrate that the test provides a sensitive and accurate plasma-based NGS method for assessing genomic alterations and immunotherapy biomarkers in a single assay.
A robust, accurate and cost-effective detection of alpha-thalassemia in an expanded multigene carrier testing
Authors: S. Kyriakou, M. Georgiadou, A. Achilleos, C. Lemesios, C. Savva, C. Havadjia, K. Tsangaras, G. Billioud, C. Sotiriou, L. Constantinou, H. Kkoufou, Lygia Ioannou, M. Spyrou, S. Pissaridou, A. Matsentidou, C. Prokopi, M. Vaki, S. Georgiou, E. Kypri, M. Ioannides, G. Koumbaris, P. C. Patsalis
Background/Objectives
Thalassemia, a group of inherited blood disorders with carrier frequency of up to 5% worldwide, exhibits a variable clinical manifestation. Due to the disease severity and high carrier frequency, thalassemia premarital screening is implemented in several countries; however, the techniques used vary and have several limitations. NGS-based technologies enable simultaneous detection of multiple genes and are highly scalable, however alpha thalassemia screening via NGS is challenging due to the high homology in the HBA gene cluster region.
Our aim is to validate a targeted, NGS-based, in-solution hybridization protocol for the detection of alpha thalassemia, so as to simultaneously detect multiple inherited conditions in our carrier screening test.
Methods
We utilized a proprietary targeted, NGS-based, in-solution hybridization assay that covers all coding regions and exon-intron boundaries of 230 genes related to autosomal recessive and X-linked inherited conditions. An in-house algorithm was designed and used on 200 individuals of known carrier status. Subsequently, a blind validation study was performed on 202 individuals of unknown variant status and individuals who carry an alpha thalassemia deletion previously identified by an independent laboratory, to set the sensitivity and specificity of alpha thalassemia detection. The assay of the proprietary technology is described here.
Results
- Sensitivity and specificity for SNVs and indels was 100% with 95% confidence interval (CI).
- The specificity and sensitivity of CNVs was 100% and 93%, respectively.
- The alpha thalassemia copy number changes (deletions and amplifications) ratio in the 202 samples of the validation study was observed.
Conclusion
NGS is an efficient technology that helps detect missed thalassemia carriers, as well as identify unknown mutations that are typically undetected by routine analysis, and allows for large-scale genetic screening. This is a robust, accurate, scalable, and cost-effective end-to-end CE-IVD solution that eliminates the need for additional testing for alpha thalassemia.
Novel variants in the PKD1 and PKD2 genes in German patients with autosomal dominant polycystic kidney disease
Authors: R. Zarbock, K. Mayer, M. Scholz, J. Philippou-Massier, K. Hörtnagel, I. Rost
Background/Objectives
Our team recorded the results of testing for autosomal dominant polycystic kidney disease (ADPKD), a potentially fatal hereditary disease with a prevalence of 1:1000 whose molecular diagnosis is hampered by high allelic heterogeneity and the presence of six highly homologous PKD1 pseudogenes.
Though over 1300 different causative variants for ADPKD are found in PKD1 or PKD2 genes, causative variants in other genes, such as GANAB and DNAJB11, have been discovered in rare cases. Despite being a dominant disease, multiple variants in one patient may exacerbate the clinical phenotype.
Methods
119 individuals, both males and females, with suspected ADPKD were analyzed as part of routine diagnostic testing over a 36-month period. Detection of variants in ADPKD-associated genes was performed by targeted NGS sequencing on Illumina platforms, and long-range PCR followed by Sanger sequencing was also used for PKD1 testing. NGS and MLPA were used for CNV detection.
Results
- 61.3% of patients were diagnosed with ADPKD.
- Of the 78 variants identified, 70 were in the PKD2 gene and 8 in the PKD1 gene. According to ACMG guidelines, 40 variants were classified as pathogenic, 12 as likely pathogenic and 21 as VUS.
- The majority of mutations were missense (41%), and the rest were frameshift (26%), nonsense (16%), splice (10%), deletion (4%) and in-frame (3%).
- 5 patients, most of whom were diagnosed at a younger age than the rest, had more than one affected variant.
- 40 of the variants detected were novel.
- All PKD1 variants identified by Sanger sequencing were also detected by NGS, despite reports that the presence of pseudogenes can interfere with NGS. However, Sanger sequencing still proved to be valuable due to poor NGS coverage in certain PKD1 exons.
Conclusion
Our results highlight the high allelic heterogeneity of ADPKD variants, which is further complicated by the presence of VUS. The discovery of 40 previously undescribed variants has significantly expanded the spectrum of known ADPKD causative variants.
Development of a universal assay for multiple targeted NGS tests supported by a novel sample data management tool
Authors: S. Kyriakou, M. Georgiadou, A. Achilleos, C. Lemesios, C. Savva, C. Havadjia, K. Tsangaras, G. Billioud, C. Sotiriou, L. Constantinou, H. Kkoufou, L. Ioannou, M. Spyrou, S. Pissaridou, A. Matsentidou, C. Prokopi, M. Vaki, S. Georgiou, E. Kypri, M. Ioannides, G. Koumbaris, P. C Patsalis
Background/Objectives
Our aim is to develop a scalable, cost- and time-efficient, single workflow solution that meets the needs of laboratories of any size, utilizing our proprietary technology platform for the identification of genetic variants in multidisciplinary fields.
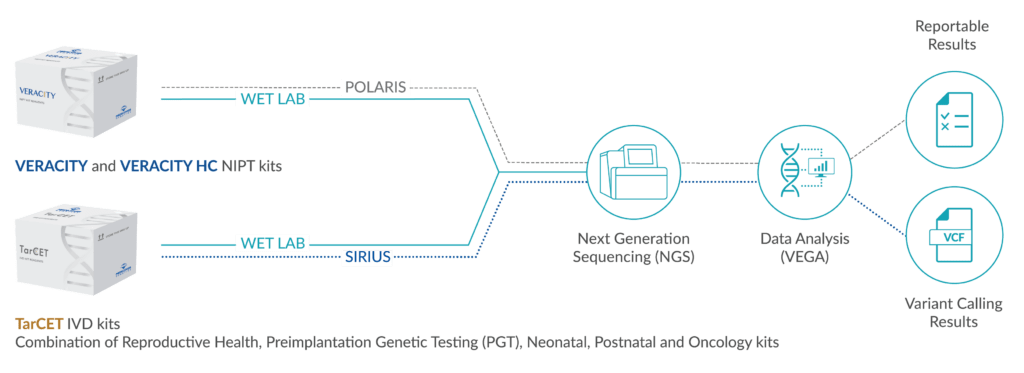
Our NGS-based workflow solution utilizes a single assay for all tests, and a streamlined workflow through single-tube library preparation. It is compatible with Illumina sequencing platforms, has automation compatibility to reduce user errors, and provides an advanced data management system and multiple result interpretation forms. The 13 TarCET IVD kits, which are utilized in the streamlined workflow, cover multiple genes in the reproductive health, preimplantation genetic testing, neonatal, postnatal, and oncology fields.
Methods
Between 16 and 57 samples were tested per kit, with the samples being:
- Individuals of unknown variant status. An orthogonal method was used to confirm every positive call.
- Individuals who were carriers of genetic disorders, as previously identified by an independent laboratory.
TarCET Kits Workflow
- DNA preparation depending on original sample type
- Universal library preparation
- Universal capture enrichment
- Universal elution and post capture amplification
- Universal sample loading and sequencing
Sirius Data Management Software: A genetic data management web-based application that enables users to manage information generated by NGS analysis after sample processing through TarCET kits workflow.
Results
Analytical concordance between our assays and other orthogonal methods or independent laboratories demonstrated 100% sensitivity with a CI of 98-100%, and 100% specificity with a CI of 99.9-100%, for both SNVs and indels. The algorithm was designed to detect CNVs at a few exon resolution with high sensitivity and specificity.
Conclusion
We have developed and validated a comprehensive, streamlined workflow that allows for the simultaneous analysis of multiple tests in a single sequencing run providing fast and reliable results.
Genetic diagnostic approach in nephrotic syndrome to decide on treatment strategy
Authors: V. Chini, S. Sevastidou, N. Dounavi, S. Karapanou, C. Pangalos, V. Velissariou
Background/Objectives
Nephrotic syndrome is divided into two categories depending on response to treatment: steroid-sensitive and steroid-resistant. As the name indicates, the steroid-resistant type is resistant to steroid treatment and therefore progresses to end-stage renal failure in the first or second decade of life.
The disorder is autosomal recessive and is characterized by childhood onset of proteinuria, chronic kidney disease signs, and focal segmental glomerulosclerosis, all of which might be symptoms of multiple disorders, therefore extended WES analysis is necessary for definitive clinical diagnosis and appropriate management.
Methods
39-year old male, who has been presenting with symptoms associated with nephrotic syndrome since 9 years old and has been receiving cortisone treatment in the past but has subsequently stopped responding to therapy, is tested by WES through Thermo Fisher Scientific’s Ion Proton System.
Raw data alignment, variant calling and bam file analysis were performed according to the manufacturer’s software. Analysis and filtering of the annotated VCF file was performed in-house.
Results
- A mutation and a polymorphism were identified in NPHS2, in compound heterozygosity, confirming the clinical diagnosis of steroid-resistant nephrotic syndrome and enabling accurate prognosis.
- From the non-filtered VCF file of 36,060 variants, the filtered variants list identified 677 variants, of which tertiary analysis identified a heterozygous, likely pathogenic variant in NPHS2 which is associated with steroid-resistant nephrotic type 2: c.1013_1014ins (p.Val339Glyfs*).
- When variant analysis was extended in the nonfiltered VCF file, a heterozygous polymorphism, one of the most common NPHS2 variants in Caucasians was identified: c.686G>A (p.Arg229Gln, p.R229Q). Compound heterozygous patients with this variant do not respond to corticoids or immunosuppressants, and do not relapse after kidney transplantation.
- Co-existence of a pathogenic mutation in NPHS2 with p.R229Q polymorphism has been described in patients with adult-onset steroid-resistant nephrotic syndrome who exhibited slower progression to end-stage renal failure.
Conclusion
Patients with clinical diagnosis of nephrotic syndrome should be tested by WES prior to treatment in order to confirm diagnosis, receive accurate prognosis, and avoid unnecessary cortisone administration. Bioinformatic analysis should be performed without filtering to avoid missing variants which are critical for disease prognosis. In couples where one person is a carrier of an NPHS2 mutation, the other should be screened for the p.R229Q polymorphism.
Remarkably mild clinical presentation of classical Ehlers-Danlos syndrome (cEDS) in a three generation family due to a novel COL5A2 Glycine substitution
Authors: K. Mayer, M. Cohen, L. Peterson, K. Hörtnagel
Background/Objectives
cEDS, an autosomal dominant disorder, is caused by variants in several genes; COL5A1 (70%), COL5A2 (13%), and COL1A1 or other genes (10%). COL5A2 variants often lead to a more severe cEDS phenotype, but there is absence of genotype-phenotype correlations. A family with a COL5A2 mutation and a remarkably mild cEDS clinical phenotype is presented.
Methods
Pedigree shows a 54-year old grandmother, three of her six children, and her 8-year old grandchild affected by cEDS. Patient characteristics consistent with cEDS are observed and noted in the grandmother, the 31-year old mother, and her 8-year old, who is noted as ‘index patient’. To fulfill the minimal criteria suggestive of cEDS, patients should present with all major criterion 1 (MM1) characteristics, and either major criterion 2 (MM2) or 3 minor criteria (MN) characteristics as described below:
- MM1 characteristics: generalized skin hyperextensibility, extensive atrophic scarring
- MM2 characteristics: generalized joint hypermobility of BS>5, Beighton score
- MN characteristics: easy bruising, soft doughy skin, skin fragility, mucocutaneous features, hernia, epicanthal folds, complications of joint hypermobility, first degree relative who meets clinical criteria.
Results
- None of the so far tested family members carrying the p.(Gly582Val) mutation on COL5A2 fulfill the obligate minimal criteria suggestive of cEDS.
Conclusion
Clinical descriptions of cEDS cases with COL5A2 glycine substitutions are rare, with isolated cases lacking the combined MM1 characteristics. Strict application of current criteria could lead to underdiagnosis, thereby publishing detailed genotype-phenotype characterizations will hopefully contribute to future updates of the EDS nosology concerning the significance of skin hyperextensibility/atrophic scarring.