Infertility is a major public health issue with approximately 1 in 6 people worldwide suffering from infertility during their reproductive lifespans [1]. It is defined as the failure to achieve a pregnancy after ≥ 12 months of regular unprotected sexual intercourse [WHO]. Both male and female reproductive systems can be affected.
Currently, the diagnostic timeline of infertile couples includes biochemical and instrumental analyses that allow for a diagnosis in 65% of cases; in the remaining undiagnosed 35% of cases, genetic tests are performed. Genetic tests have three main purposes in reproductive health: the identification of the infertility causes, identification of genetic diseases transmissible to offspring, and optimization of the assisted reproductive technology [1].
Before getting into detail about what genetic tests can be performed and who should consider genetic testing, it is important to define what reproductive health is.
Contents
- What is reproductive health?
- When to take genetic tests during the reproductive journey?
- PRECONCEPTION
- PRE-IMPLANTATION
- PRENATAL
- POSTNATAL
- Who can benefit from this test
- What genetic tests can be done?
- Genetic testing before pregnancy (preconception)
- Genetic testing during pregnancy (prenatal)
- Genetic testing after pregnancy (postnatal)
- References
What is reproductive health?
According to the report of the International Conference on Population and Development (ICPD) in 1994 reproductive health is defined as follows:
“Reproductive health is a state of complete physical, mental and social well-being and not merely the absence of disease or infirmity, in all matters relating to the reproductive system and its processes. Reproductive health, therefore, implies that people are able to have a satisfying and safe sex life and that they have the capability to reproduce and the freedom to decide if, when, and how often to do so.” [2]
This implies that both men and women have the right to be informed and have access to safe, effective, affordable and acceptable methods of family planning of their choice, as well as other methods for the regulation of fertility. Additionally, women have the right to access appropriate healthcare services that enable them to go safely through pregnancy and childbirth and provide couples with the best chance of having a healthy child.
It also includes sexual health, the purpose of which is the enhancement of life and personal relations, and not merely counseling and care related to reproduction and sexually transmitted diseases.
When to take genetic tests during the reproductive journey?
Genetic testing can be performed at any stage of the reproductive journey [3].
PRECONCEPTION
Starting from preconception (before conception), genetic tests can detect genetic carriers of frequent diseases like cystic fibrosis, hemophilia (hemophilia A or hemophilia B), or fragile X syndrome (see carrier screening for more information).
Anyone can carry certain mutations (genetic changes) in their body. Some mutations may have no effect on our health and development, while others can cause a genetic disease. When an individual has a mutation in one of their genes, that individual is a carrier of a recessive disease. Two carriers of the same recessive disease can have a child who is affected if the child inherits the mutation from both of them. It is estimated that humans carry an average of one to two mutations per person that can cause severe genetic disorders or prenatal death when two copies of the same mutation are inherited [4].
Currently, there are many genetic tests that assess the “carrier status” of a patient or a couple to reduce the chances of having a baby with a genetic disorder (see carrier screening for more information). Genetic carrier screening detects mutations causing a number of recessive genetic conditions in an individual, that can be passed on to their offspring if the couple carries the mutation.
Autosomal/X-linked recessive disorders are more frequent than autosomal/X-linked dominant because the latter present a higher deleterious effect. The reproductive approach is different in these cases because patients are not only carriers, but also suffer the illness, and are aware that they can transmit the genetic condition with a 50% chance to their offspring.
PRE-IMPLANTATION
Pre-implantation can ensure a chromosomal and genetically normal embryo is transferred, thereby, decreasing the risk of monogenetic diseases like Duchenne muscular dystrophy, aneuploidies such as Down’s syndrome, or structural diseases like DiGeorge’s syndrome or Prader-Willi syndrome.
At this stage of the reproductive journey, it is useful to group together couples with known reproductive problems, e.g., infertility, miscarriages, previously affected child, as well as those couples with a family history of a genetic condition. All of them could benefit from assisted reproduction techniques such as pre-implantation genetic testing for monogenic diseases (PGT-M) and pre-implantation genetic testing for aneuploidies (PGT-A).
PGT-M allows us to detect embryos affected by a known monogenic that has been previously detected in their parents. Other genetic conditions that have an impact on fertility, pregnancy, parents, and newborns are the so-called chromosomal disorders. With increasing age, fertility declines. There is an increased risk of numerical and structural chromosomal abnormalities, which can lead to implantation failure, early pregnancy loss, greater risk of congenital disabilities, or severe chromosomal congenital diseases such as Patau syndromes. Aneuploidy ranks as the most common genetic abnormality accounting for approximately 50% of miscarriages. More than half of the embryos produced by in vitro fertilization (IVF) are aneuploid [5].
The process of detecting numeric or structural chromosomal abnormalities for the purpose of embryo selection is generally referred to as pre-implantation genetic testing for aneuploidies (PGT-A) to increase implantation and pregnancy rates, and decrease miscarriage rates and the risk of aneuploid offspring, as well as decrease the time to conceive. In recent years, PGT-A using FISH screening has been initially replaced by comprehensive approaches, including comparative genomic hybridization arrays (CGH) or single nucleotide polymorphism microarrays, and more recently, by next-generation sequencing (NGS)-based techniques. Additional genetic tests assessing fertility can include an endometrial microbiome test.
PRENATAL
Genetic analysis is also useful for prenatal diagnosis of monogenic diseases and aneuploidies, high-risk pregnancies, and in the case of spontaneous abortions, the analysis of the products of conception.
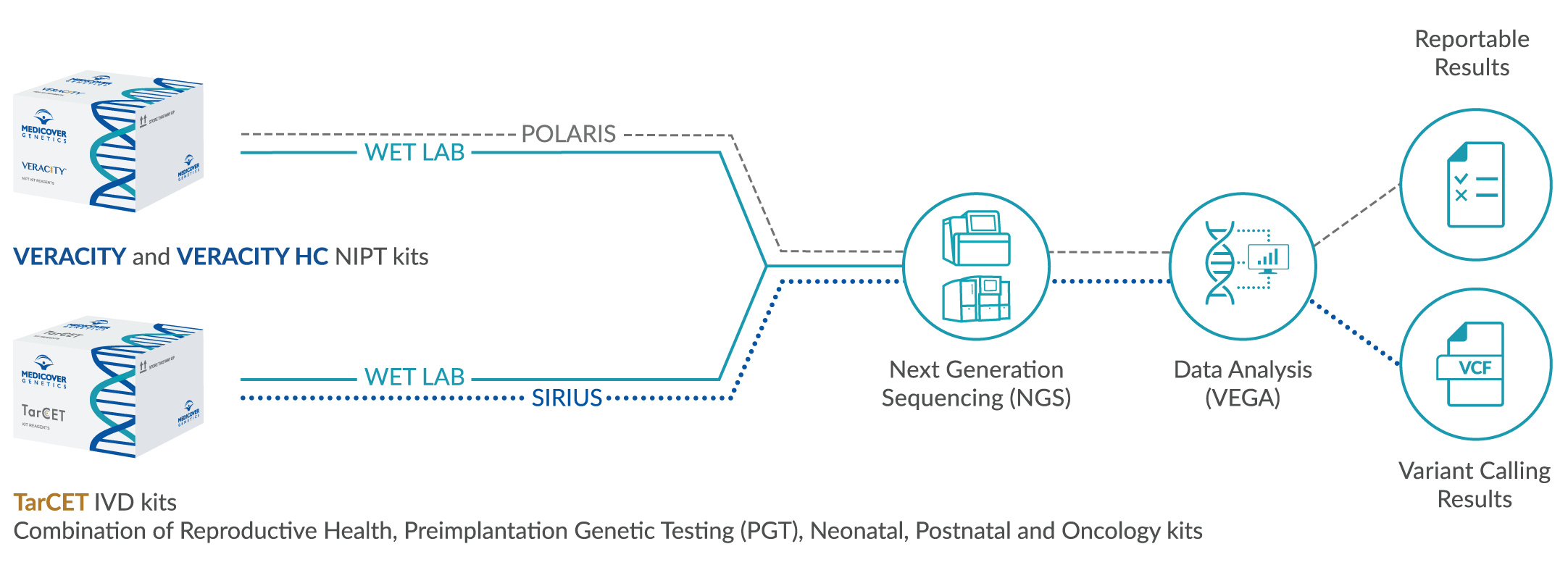
During pregnancy, fetal DNA travels from the placenta to the mother’s bloodstream and circulates along with her own DNA. A new generation of a non-invasive prenatal test, called VERACITY, can accurately measure the fetal cell-free DNA in the maternal blood to detect the presence of fetal aneuploidies and microdeletions. VERACITY has been validated for both single and twin pregnancies as well as pregnancies conceived through in-vitro fertilization (IVF).
Some of the clinical features of these monogenic disorders, especially those associated with syndromic forms, can be identified throughout pregnancy by ultrasonography analyses. In these cases, and depending on the clinical impression, specific tests can be used to analyze certain genes or variants, as well as more complex and nonspecific technologies, such as CMA, NGS gene panels, or whole exome sequencing (WES), when precise clinical guidance is not possible. In any case, when an ultrasound finding is detected during pregnancy, that pregnancy is labeled as high risk for a genetic disease.
Unfortunately, miscarriages are the most common complication during early pregnancy. Clinically recognized pregnancy losses occur in approximately 15–25% of pregnancies, most of them occurring during the first trimester. Although there are many known causes and risk factors for early pregnancy loss, about 60% of those cases are caused by sporadic chromosomal abnormalities which are usually numerical (86%). These cytogenetic anomalies include autosomal trisomies (27%), polyploidies (10%), chromosome X monosomy (9%), and structural rearrangements (2%); double trisomies, as well as multiple trisomies, which are infrequent, have an incidence of about 0.7%.
Whole exome sequencing (WES) is very useful for the detection of alterations in the sequence of any gene that may be related to the potential genetic condition that may have caused the spontaneous termination of the pregnancy in progress. This is especially important in the second trimester of gestation when monogenic disorders acquire a higher frequency. In these cases, identification of the molecular causes of the miscarriage can be very useful to prevent new similar situations in the couple.
When miscarriage occurs in advanced pregnancy, the clinical and anatomopathological evaluation of the fetus can be very useful to guide the genomic analysis. When the clinical assessment is not possible, WES provides a high capability to identify sequence variants in genes associated with complex syndromes, but also, the optimization of bioinformatic analyses, making possible the identification of copy number variations in these cases.
POSTNATAL
Lastly, genetic tests can be utilized to perform newborn screening of common and actionable diseases, personalized genetic analyses such as single gene analysis for monogenic diseases and genetic panels, or whole exome sequencing for complex or clinically unspecific diseases. In this stage, as in other previous stages, screening measures are applied to reduce the impact of congenital diseases.
Neonatal screening allows for the detection of a wide number of genetic disorders, causing health problems starting in infancy or early childhood, mainly metabolic disorders like phenylketonuria. Newborn screening programs are well-established as the standard of care in most developed countries. Early detection and treatment can help prevent inborn errors of metabolism, intellectual and physical disabilities and life-threatening illnesses during the first hours of life. The advent of next-generation sequencing has resulted in attempts to expand the use of DNA sequencing in newborn screening to improve diagnostic and prognostic utility.
Additionally, diagnostic methods can be applied in those neonates who have developed symptoms, especially for newborns admitted to the intensive care unit when disease progression is extremely rapid and a quick molecular diagnosis is relevant for clinical decision making, establishing a prognosis, defining specific therapeutic measures and providing genetic counseling and access to family studies aiming to reduce the risks of recurrence in the family. Monogenic diseases have a high impact on neonatal morbimortality, accounting for ~20% of infant deaths and ~18% of pediatric hospitalizations. Genomic testing of these patients aims to provide a comprehensive molecular diagnosis that allows for early intervention of the patient and proper genetic counseling of the family in order to reduce the time spent in the diagnostic odyssey. These tests provide a high clinical utility and are cost-effective, especially in patients involved in neonatal intensive care units.
Who can benefit from this test
- People interested in their carrier status
- Couples trying to conceive for >12 months
- Couples performing IVF
- Pregnant women
- Parents of rare disease children (unknown origin)
What genetic tests can be done?
We have an extensive portfolio of diagnostic and predictive testing, using several different technologies, that can accompany couples interested in conceiving, pregnant women and parents during their reproductive journey and early parenthood.
Genetic testing before pregnancy (preconception)
- Endometrial microbiome analysis
- Halosperm test
- Carrier screening
- Genetic infertility diagnostics
Genetic testing during pregnancy (prenatal)
- Rhesus factor (non-invasive test)
- Karyotyping, Microarray CGH
- Gene/Panel diagnostics
- Whole exome sequencing
Genetic testing after pregnancy (postnatal)
References
[1] Cariati F et al. The evolving role of genetic tests in reproductive medicine. J Transl Med. 2019 Aug 14;17(1):267. doi: 10.1186/s12967-019-2019-8. PMID: 31412890; PMCID: PMC6694655. https://translational-medicine.biomedcentral.com/articles/10.1186/s12967-019-2019-8
[2] United Nations. Report of the International Conference on Population and Development 1994. Retrieved 7 June 2022 from https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/icpd_en.pdf
[3] Garcia-Herrero S et al. The Reproductive Journey in the Genomic Era: From Preconception to Childhood. Genes (Basel). 2020;11(12):1521. Published 2020 Dec 19. doi:10.3390/genes11121521. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7767043/
[4] Gao Z et al. An estimate of the average number of recessive lethal mutations carried by humans. Genetics. 2015 Apr;199(4):1243-54. doi: 10.1534/genetics.114.173351. Epub 2015 Feb 18. PMID: 25697177; PMCID: PMC4391560. https://academic.oup.com/genetics/article/199/4/1243/5935883
[5] Rubio C et al. In vitro fertilization with preimplantation genetic diagnosis for aneuploidies in advanced maternal age: a randomized, controlled study. Fertil Steril. 2017 May;107(5):1122-1129. doi: 10.1016/j.fertnstert.2017.03.011. Epub 2017 Apr 19. PMID: 28433371. https://www.fertstert.org/article/S0015-0282(17)30254-6/fulltext