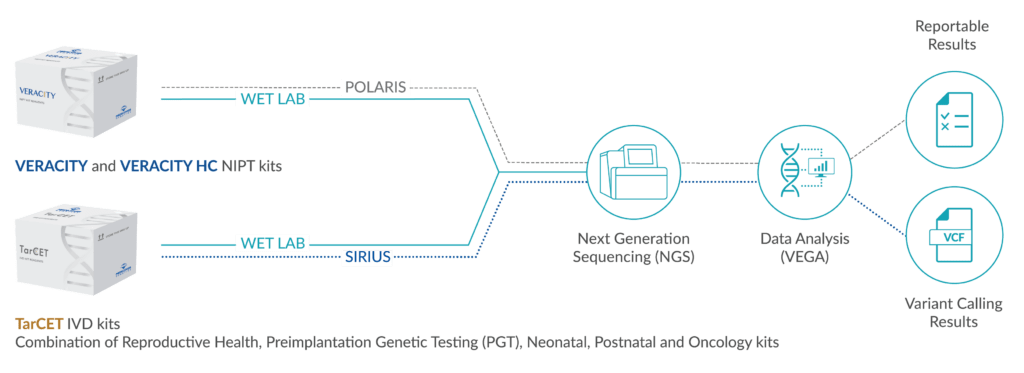
Autism spectrum disorders (ASD) are among the neuropsychiatric diseases with the greatest heritability, as shown by high concordance rates of about 70% in monozygotic twins in earlier twin studies. Based on this, the empirical risk of recurrence for siblings of children with ASD is between 5 and 20%, i.e., significantly higher than for other multifactorial diseases. ASD are both clinically and genetically heterogeneous; the most common comorbidities are developmental disorders or reduced intelligence (about 70%), speech disorders (about 30%) or epilepsy.
It is now believed that modern genetic testing methods can find a genetic cause for about 20 to 25% of autism spectrum disorders (Baker et al, 2015). Approximately 20% carry de novo generated CNV (copy number variation), which are detected by chromosomal microarrays (CMA). Monogenic causes are found in 3-5%, mostly for syndromes caused by pathogenic variants in single genes that show ASD as a partial symptom.
The American College of Medical Genetics (ACMG) (Schaefer et al, 2013) recommends the use of a chromosomal microarray for the genetic diagnosis of patients with ASD in whom clinical examination does not give rise to suspicion of a specific genetic syndrome. If the chromosomal microarray analysis is not conclusive, the MECP2 gene (Rett syndrome) should be examined in girls, the FMR1 gene (fragile X syndrome) in boys, or the PTEN gene (Bannayan-Riley-Ruvalcaba syndrome) if macrocephaly is present .
It is now assumed that numerous genes, probably more than 1000, may be involved in the development of ASD, although it is not yet clear to what extent individual variants influence the expression in individual cases. Nevertheless, advanced diagnostics using NGS (gene panel diagnostics) can also lead to a diagnosis in individual cases and thus to more precise statements on the prognosis and the risk of recurrence.
References
Grove et al. 2019, Nat Genet 51:431 / Yuen et al. 2017, Nat Neurosci 20:602 / Baker et al. 2015, Pediatr Clin N Am 62:607 / Schaefer et al. 2013, Gen Med 15(5):399
CHARGE SYNDROME
CHARGE syndrome (Hall-Hittner syndrome) is inherited in an autosomal dominant manner, but in most cases it occurs sporadically with a frequency of about 1:10,000. The acronym CHARGE stands for coloboma, heart defects, atresia choanal, growth retardation, genital hypoplasia and ear abnormalities. According to Verloes (2005), the main criteria are colobomas, choanal atresia and semicircular canal hypoplasia, while secondary criteria are functional disorders of the brain stem, such as paresis in the area of the VII to VIII cranial nerves, deafness, disturbances of the hypothalamus-pituitary axis, which includes growth hormone and gonadotropins, abnormalities of the outer and middle ear, malformations of mediastinal organs, such as the heart and esophagus and intellectual disability. The diagnosis is correct if three main criteria or two main and two secondary criteria are present. There is wide clinical variability. Life expectancy depends on the severity of the abnormalities; up to one third of those affected die within the first six months of life. In most cases there is a psychomotor developmental delay, although occasionally intelligence is within the normal range.
Pathogenic variants in the CHD7 gene are found in 60 to 70 % of patients. (CHD: chromodomain, ATPase/helicase domain and a DNA-binding domain). CHD proteins, which belong to the family of chromatin remodeling factors, influence chromatin structure and gene expression and thus have an important function in embryonic development. The variants extend over the entire coding region (exons 2-38) of the CHD7 gene. In the majority of cases, they are truncating variants that lead to a premature termination of protein synthesis. In 1-2% of the cases there are deletions. There are no genotype-phenotype correlations. In at least one case, a germ cell mosaic has been detected, so that even where there in no evidence of a variant in the parents, a low risk of recurrence cannot be excluded.
References
Moccia et al. 2018, Genet Med 20:1022 / Butcher et al. 2017, Am J Hum Genet 100:773 / Hale et al. 2016, Am J Med Genet A 170:344 / Hsu et al. 2014, J Paediatr Child Health 50:504 / Schulz et al. 2014, Hum Genet 133:997 / Pauli et al. 2009, Clin Genet 75:473 / Wincent et al. 2009, Eur J Med Genet 52:271 / Sanlaville et al. 2007, Eur J Hum Genet 15:389 / Blake et al. 2006, OJRD 1:34 / Aramaki et al. 2006, J Pediatr 148:410 / Jongmans et al. 2006, J Med Genet 43:306 / Lalani et al. 2006, Am J Hum Genet 78:303 / Verloes et al. 2005, Am J Med Genet 133A:306 / Vissers et al. 2004, Nat Genet 36:955
PITT-HOPKINS SYNDROME
Pitt and Hopkins first described two patients with a developmental disorder, similar appearance and hyperventilation seizures in 1978. From 2007, microdeletions in the 18q21.2 region and then pathogenic variants in TCF4 were reported to cause Pitt-Hopkins syndrome (PTHS).
The following external features are characteristic and become more pronounced with age: deep-set eyes slanting slightly upward and outward, prominent nasal root, slightly curved nasal bridge and flattened nasal tip, flared nostrils, short philtrum, and a prominent chin. Fetal fingertip pads are often present.
Muscle hypotonia is seen in the first year of life and most children are described as quiet during this period. A severe global developmental disorder is usually present. On average, affected children learn to walk between the ages of 3 and 4. Speech development is severely impaired or absent with many affected individuals knowing only a few words. Their mood is usually described as cheerful. Repetitive hand movements such as waving or clapping are frequently observed. About 60% have episodes of hyperventilation and/or apnea while awake that are not related to epileptic activity and almost half of the patients have epilepsy. Severe congenital malformations are rare, and chronic constipation is often present. Growth is usually unaffected, and about one-third develop microcephaly. MRI may reveal a corpus callosum deficiency and ventricular dilation. Half of the children have myopia or strabismus.
Differential diagnoses include Angelman syndrome and Mowat-Wilson syndrome as well as Rett syndrome and Joubert syndrome.
The prevalence has been estimated at 1:11,000; however, PTHS is probably underdiagnosed.
The disorder is caused by haploinsufficiency of the TCF4 gene in 18q21.2 due to pathogenic variants (approximately 70%) and deletions (approximately 30%). The TCF4 gene has 20 exons, 18 of which are protein-coding (2-19). The TCF4 protein is a transcription factor that is highly expressed in embryonic development, including in the CNS.
As pathogenic variants and deletions in TCF4 usually occur de novo, the recurrence risk for siblings is low unless a somatic or germ cell mosaic has been detected in one parent.
References
Goodspeed et al. 2018, J Child Neurol 33:233 / Whalen et al. 2012, Hum Mut 33:64 / Ardinger et al. 2012, GeneReviews™ / Marangi et al. 2011, Am J Med Genet 155A:1536 / Amiel et al. 2007, Am J Hum Genet 80:988 / Zweier et al. 2007, Am J Hum Genet 80:994
RUBINSTEIN-TAYBI SYNDROME
Rubinstein-Taybi syndrome (RTS) is characterized by symptoms of low intelligence, postnatal growth delay with reduced final height and microcephaly. Distinctive facial features are seen, such as a deep hairline, broad, arched eyebrows, outwards and downward sloping eyes, base of the columella below the nostrils when seen in profile, retrognathia, and dental anomalies. Additionally, broad, often radially angled thumbs and broad big toes are seen. When laughing, there is a characteristic facial expression with almost closed eyes. Seizures occur frequently. RTS usually occurs sporadically, and the prevalence is estimated at approximately 1:100,000‑125,000.
Pathogenic variants in the CREBBP gene, which codes for the cyclic AMP-regulated enhancer binding protein, are a known cause of RTS (approximately 50-70%). In addition, pathogenic variants have been described in the EP300 gene (approximately 5%), which codes for the E1A binding protein p300. These two proteins are transcriptional co-activators involved in many signaling pathways within the cell (e.g., DNA repair, growth, differentiation and apoptosis).
References
Korzus 2017, Adv Exp Med Biol 978:39 / Fergelot et al. 2016, Am J Med Genet A 170: 3069 / Milani et al. 2015, Pediatr 41:4 / Stevens et al. 2014, Gene reviews / Bartsch et al. 2010, Am J Med Genet 152A:181 / Schorry et al. 2008, Am J Med Genet 146A:2512
SOTOS AND WEAVER SYNDROME
Sotos syndrome is an overgrowth syndrome of childhood that is characterized by macrocephaly, distinctive craniofacial features, mild intellectual disability (IQ average 76), advanced bone age, and normal height in adulthood. The head is narrow and long (dolichocephalic), the forehead high and broad with a laterally receding frontal hairline, and the is chin accentuated and pointed. The distance between the eyes seems widened, and the eyelids slope downwards. The palate is pointed and high. Hands and feet are large and the joints are often hyperextensible. Feeding problems are common in infancy. About 50% of the children have seizures, and about half of them occur with fever. Congenital malformations such as heart defects are rare. A slightly increased tumor rate has been reported involving various tissues. Increased anxiety, hyperactivity and aggressiveness are often described.
In 75-90% of cases, Sotos syndrome is caused by nucleotide alterations or deletions in the NSD1 gene (nuclear receptor-binding SET domain-containing protein 1) in 5q35. In Central Europe and the USA, 60-80% of patients carry nucleotide changes and about 10% carry deletions, while in Japanese patients, microdeletions are causal in over 50%. The frequency of Sotos syndrome is estimated at 1:10,000 to 1:50,000. The recurrence risk for siblings is low because the nucleotide changes or deletions are mostly new.
Weaver syndrome (WVS; OMIM 277590), a rare autosomal dominant disorder characterized by tall stature, variable intellectual disability and characteristic facial dysmorphia, offers a potential differential diagnosis. Pathogenic variants in the EZH2 gene (enhancer of zeste, Drosophila, homolog 2; OMIM 601573) have been found to be causative for a large number of Weaver syndrome cases. However, genetic heterogeneity cannot be excluded.
References
Lane & Freeth 2019, Chromatin Signaling and Neurological Disorders, Vol 7, pp. 219 / Tatton-Brown et al., Sotos Syndrome. 2004 Dec 17 [Updated 2019 Aug 1]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2021 / Imagawa et al. 2017, Hum Mutat 38:637 / Leventopoulos et al. 2009, Pediatr Neurol 40:357 / Tatton-Brown et al. 2007, Eur J Hum Genet 15:264 / Baujat et al. 2007, Orphanet J Rare Dis 2:36 / Visser et al. 2005, Am J Hum Genet 76:52 / Rio et al. 2003, J Med Genet 40:436 / Kurotaki et al. 2002, Nat Genet 30:365